verifynow test package insert|verifynow werfen : manufacture The VerifyNow-P2Y12 Assay/VerifyNow PRU Test is a rapid test that uses ADP to stimulate platelets in the presence of PGE1 [Prostaglandin E1] and which inhibits activation downstream .
webReviews. Videos. Photos. About. See all. Avenida Fernando Machado, 3939D 89805203 Chapecó, SC, Brazil. O Jump World Park é um parque indoor em Chapecó que nasceu com o intuito de reunir toda a família e amigos em um espaço seguro, com muita diversão, prazer e interação! 807 people like this. 826 people follow this. 257 people checked in here.
{plog:ftitle_list}
The latest tweets from @__Dioguinho
werfen verify now
moisture meter still shows dry
verifynow werfen
Test Specificity The VerifyNow PRUTest is formulated to have greater specificity for platelet aggregation mediated by the P2Y12 receptor than the P2Y1 receptor, both of which are .VerifyNow PRUTest* Measures the level of platelet P2Y12-receptor blockade to help identify patient response to antiplatelet therapy, including clopidogrel (Plavix), prasugrel (Effient) and ticagrelor (Brilinta).VerifyNow A platelet function test developed by Accumetrics. The VerifyNow system is a whole blood, point-of-care assay, which consists of a turbidimetric-based optical detection instrument, single-use assay devices, and associated .
Discover and assess platelet reactivity to anti-platelet medications, such as Aspirin and clopidogrel in minutes with Werfen's VerifyNow platelet testing system.VerifyNow Aspirin Test. Utilizes an arachidonic acid-initiated reaction to measure platelet response to aspirin. Commonly prescribed antiplatelet medications, including clopidogrel .The VerifyNow-P2Y12 Assay/VerifyNow PRU Test is a rapid test that uses ADP to stimulate platelets in the presence of PGE1 [Prostaglandin E1] and which inhibits activation downstream .
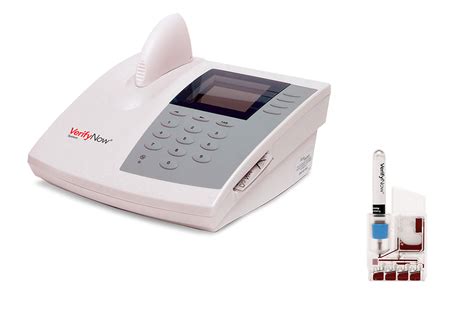
The VerifyNow Aspirin Test is a qualitative test to aid in the detection of platelet dysfunction due to aspirin ingestion in citrated whole blood for the point of care or laboratory setting.For more details, see the VerifyNow Test package insert. 1 Plavix (clopidogrel bisulfate) tablets Prescribing Information. Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership. 3/2010
moisture meter still shows dry plants
VerifyNow, the most cited and utilized FDA-cleared platelet reactivity test, may help identify patients at risk of a major adverse cardiac event (MACE) due to poor drug response. .VerifyNow PRUTest Measures the level of platelet P2Y12-receptor blockade to help identify patient response to antiplatelet therapy, including clopidogrel (Plavix), prasugrel (Effient) and ticagrelor (Brilinta).VerifyNow Aspirin Test. Aids in the assessment of Aspirin's affect on platelets, allowing rapid, informed treatment decisions. VerifyNow PRUTest* Measures the level of platelet P2Y12-receptor blockade to help identify patient response to .Control section of this package insert for additional information. • If drawing blood for a CBC at the same time as sample collection for VerifyNow Aspirin Test, fill the CBC tube last. • Do not freeze or refrigerate sample. • Collection of the blood sample should be performed with care to avoid hemolysis or contamination by tissue factors.

Package insert: VerifyNow P2Y12, 14353.D. Accumetrics, Inc, 04/27/2009. Specimen Requirements. . Test Set Up. Monday through Sunday Turnaround Time: STAT: not available Routine: 2-4 hours after receipt in laboratory. Test Classification and CPT Coding. 85576. Reference Values. Prasugrel and ticagrelor are potent oral platelet P2Y12 inhibitors and are recommended over clopidogrel in patients with acute coronary syndrome (ACS). Oral platelet P2Y12 inhibitors are characterized by varying degrees of pharmacodynamic response profiles as assessed by a variety of commercially available assays. Because of its ease of use, rapid .VerifyNow Aspirin Test measures a patient's platelet response to Aspirin, while VerifyNow PRUTest* helps identify patients responsive to antiplatelet therapy. . Package size Part number Download VerifyNow Aspirin Platelet Reactivity Test. 00085053 Search documents (login required) > VerifyNow Assay WQC. 00085047 Search documents (login .
The VerifyNow® Aspirin Test is a qualitative test to aid in the detection of platelet dysfunction due to aspirin (ASA) ingestion in citrated whole blood for the point of care or laboratory setting. The VerifyNow® System is a turbidimetric based optical detection system which measures platelet induced aggregation as an increase in light .
VerifyNow Aspirin Test measures a patient's platelet response to Aspirin, while VerifyNow PRUTest* helps identify patients responsive to antiplatelet therapy. . Package size Part number Download VerifyNow Aspirin Platelet Reactivity Test. 00085053 Search documents (login required) > VerifyNow Assay WQC. 00085047 Search documents (login .
The VerifyNow PRU Test (CPT 85576) is designed to measure P2Y12 receptor blockade. Results of the PRU Tests are reported as P2Y12 Reaction Units (PRU). PRU measures the extent of platelet aggregation in the presence of a P2Y12 inhibitor Lower PRU levels are associated with expected antiplatelet effect. <180 PRU – suggests P2Y12 inhibitor effect
According to the VerifyNow package insert, the highest combination of sensitivity and specificity for identifying a measureable effect of a P2Y 12 inhibitor is a PRU of 208. To date, cut-off values have mainly been investigated in patients undergoing PCI.
VerifyNow P2Y12 Platelet Reactivity Test [Package Insert]. Accumetrics, San Diego, CA, 2013. has been cited by the following article: TITLE: Implications of the VerifyNow P2Y12 Assay on Patient Outcomes. AUTHORS: Denise A. Sutter, Gregory S. King, Marintha R. ShortTyping either of these before a search term will limit results to a particular name or test code. name:Kit; code:RMISCThe VerifyNow package insert instructs the user to test blood samples within 4 hours after blood draw. Three stability studies are in progress to evaluate the stability of VerifyNow P2Y12 assay devices. The first study is being performed to determine the shelf life of VerifyNow P2Y12 assay devices when stored at recommended storage temperature.The VerifyNow System is intended for use with human whole blood and VerifyNow Test Devices. The VerifyNow System should be operated by health care professionals trained on use of the system and in accordance with institution policies and procedures. . Refer to the VerifyNow Aspirin package insert for information to be considered for patients .
The VerifyNow platform is one of the most user friendly point-of-care platelet function test systems because it produces rapid results at the patient bedside. The purpose of the present paper is to give insight into the principal mechanisms of the VerifyNow system, to discuss its clinical utility for the monitoring of antiplatelet therapy and .
VerifyNow, the most cited and utilized FDA-cleared platelet reactivity test, may help identify patients at risk of a major adverse cardiac event (MACE) due to poor drug response . VerifyNow will tell you whether or not the antiplatelet medication you prescribed is safe and effective for your patient It is an important tool among leaders .Open instrument cover. Insert the test device at the instrument prompt. If this is a new device lot, the Bar Code prompt will display. . 2 if further service is required. References: 1. VerifyNow Assay WQC, Package Insert, PN 14349 2. .Heart Health Test Kit VerifyNow® PRUTest™ P2Y12 Assay 25 Tests CLIA Non-Waived TEST KIT, VERIFYNOW PRU (25TST/BX) Product Images Compare . Features. The VerifyNow PRUTest is a whole blood test used in the laboratory or point-of-care setting to measure the level of platelet P2Y12 receptor blockade Each test device contains lyophilized .
VerifyNow Aspirin Test measures a patient's platelet response to Aspirin, while VerifyNow PRUTest* helps identify patients responsive to antiplatelet therapy. . Package size Part number Download VerifyNow Aspirin Platelet Reactivity Test. 00085053 Search documents (login required) > VerifyNow Assay WQC. 00085047 Search documents (login . The introduction of foreign bodies into the blood stream, injury to the vessel wall, and plaque disruption are factors that lead to platelet activation during endovascular procedures, 1,2 as has been shown in multiple studies performed in patients undergoing coronary or cerebral angioplasty. 3,4 Angioplasty and stent placement are associated with an increased risk of .
10. VerifyNow PRUTest [package insert]. San Diego, CA; Accriva Diagnostics. 11. VerifyNow Aspirin Test [package insert]. San Diego, CA; Accriva Diagnostics. “Testing with VerifyNow supports ongoing expansion of the frontiers of endovascular aneurysm treatment by helping minimize risk of perioperative complications. ” Josser E. Delgado, MD
10. VerifyNow PRUTest [package insert]. San Diego, CA; Accriva Diagnostics. 11. VerifyNow Aspirin Test [package insert]. San Diego, CA; Accriva Diagnostics. “Testing with VerifyNow supports ongoing expansion of the frontiers of endovascular aneurysm treatment by helping minimize risk of perioperative complications. ” Josser E. Delgado, MD
Werfen in Dubai The One Tower - Office 3006, Barsha Heights Tecom, Dubai - UAE P.O. Box 117492 Phone: +971 45 543 284
VerifyNow Aspirin Test, Package Insert, PN 14438 VerifyNow System User Manual, PN 14439.C Version dated 3/2013 Portions ©2024 Mayo Foundation for Medical Education and Research.VerifyNow Platelet Inhibition Lab Code VERPLT Epic Ordering VerifyNow Platelet Inhibition Description. Results could be affected by the following: GPIIb/IIIa inhibitors, low HCT, low PLT or inherited platelet disorders. Patients with HCT<20% or PLT<50 K/uL cannot be tested with the VerifyNow Assay. . This test is not to be used for .Epic Code LAB3326 VerifyNow® P2Y12 assay for Antiplatelet Effect Important Note. 5/28/21 There is currently a shortage of this tube. Please contact Phlebotomy Services for tube availability prior to ordering the VerifyNow Assay. . Due to the special handling and time sensitivity, this test will ONLY be drawn on. INPATIENTS: Spectrum Health .
Accumetrics VerifyNow-Aspirin Assay (Formerly Ultegra RPFA-ASA) Accumetrics 3985 Sorrento Valley Blvd. San Diego, CA 92121 . Test Result Aspirin State Present Absent Positive (<550 ARU) 245 0 Negative . Please see package insert for additional information. 0050. As mentioned previously, according to the VerifyNow package insert, the highest combination of sensitivity and specificity for identifying a measureable effect of a P2Y 12 inhibitor is a PRU of 208. However, among clinical studies, a consensus has not yet been reached on optimal cut-off values. . VerifyNow P2Y12 Platelet Reactivity Test .

Resultado da 20 de nov. de 2020 · Os programas sociais do Governo Federal evitaram que a pobreza no país subisse quatro pontos percentuais durante a crise da .
verifynow test package insert|verifynow werfen